
So, it also has three sigma bonds and no lone pairs. Give the hybridization for the Br in BrF5. Determine the molecular geometry at each of the 2 labeled carbons. This corresponds to sp2 hybridization.įor the second carbon atom (CH), it forms one single bond with the first carbon atom, one single bond with the hydrogen atom, and one single bond with the oxygen atom. Identify the number of electron groups around a molecule with sp2 hybridization.

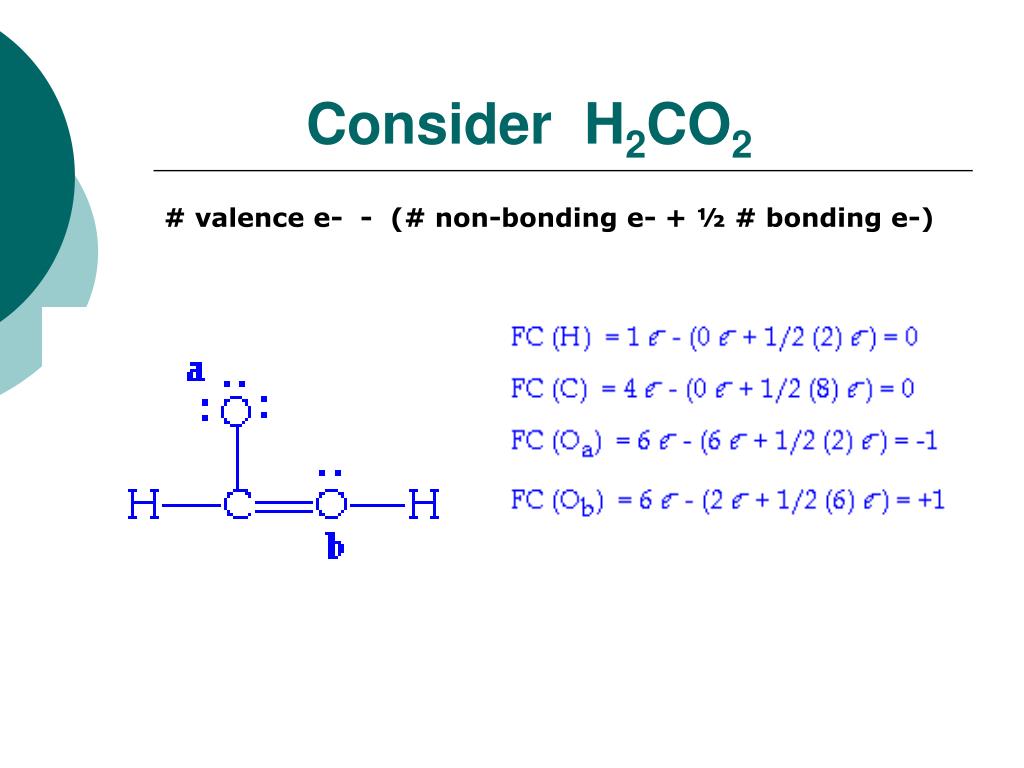
So, it has three sigma bonds and no lone pairs. So, the formal charge of the carbon atom in H2CO is +1.įor Question 2, we need to find the hybridization of the two carbon atoms in H2C-CH-OH.įor the first carbon atom (H2C), it forms two single bonds with two hydrogen atoms and one single bond with the second carbon atom. So, there are no non-bonding electrons and 6 bonding electrons (2 from each single bond and 4 from the double bond).įormal Charge of Carbon = 4 - 0 - (1/2 * 6) = 4 - 3 = 1
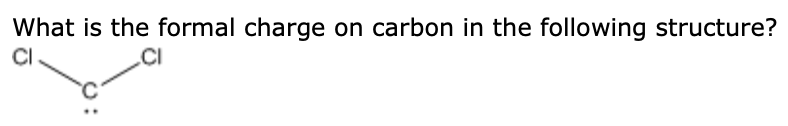
carbon atoms, one oxygen atom), even though both can engage in hydrogen C. In H2CO, carbon forms two single bonds with two hydrogen atoms and one double bond with one oxygen atom. CH3OH Draw the best Lewis structure for CBrzt What is the formal charge on the C. By assigning formal charges, we can ensure that the Lewis structure represents the correct electron arrangement. The formula for formal charge is:įormal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)Ĭarbon has 4 valence electrons. CO2, how many bonds are between the carbon and two oxygens 4 1 2 3, The attraction of the positive end of one molecule for the negative end of a. In the Lewis structure of formaldehyde, each atom should have a formal charge of zero.The formal charge is a way to determine the distribution of electrons in a molecule. Therefore, the above lewis structure of H2CO is most appropriate, reliable, and stable in nature because the overall formal charge is zero.
Formal charge of carbon in h2co download#
For Question 1, we need to find the formal charge of the carbon atom in H2CO (formaldehyde). Read Or Download Gallery of c2h2 lewis structure valence electrons formal charge - C2h2. So, each atom(oxygen, hydrogen, and carbon) in the H2CO lewis structure gets a formal charge equal to zero.


 0 kommentar(er)
0 kommentar(er)
